

Calculation and Conversion to Pauling Units." Journal of Chemical Education, volume 65, number 1, 1988, pp. 34–41. "Revised Mulliken Electronegativities: I. "Atomic and Group Electronegativities from the Electron-Density Distributions of Molecules." Journal of the American Chemical Society, volume 110, number 13, 1988, pp 4182–4186. London: Academic Press, Inc., 1979.īoyd, Russell J., and Kenneth E. "Van der Waals Volumes and Radii." The Journal Secondary Reference Points." Metrologia, volume 33, number 2, 1996, pp. 133–154. "Recommended Values of Temperature on the International Temperature Scale of 1990 for a Selected Set of "Reevaluation of X-Ray Atomic Energy Levels." Reviews of Modern Physics, volume 39, "Van der Waals Radii of Elements." Inorganic Materials, volume 37, number 9, 2001, pp. 871–885. "Binding Energies in Atomic Negative Ions: III." Journal of Physical and Chemical Reference Data, volume 28, number 6, 1999, pp. 1511–1533.īatsanov, S. "Abundances of the Elements: Meteoritic and Solar." Geochimica et Cosmochimica Acta, "A Scale of Electronegativity Based on Electrostatic Force." Journal of Inorganic and NuclearĬhemistry, volume 5, number 4, 1958, pp. 264–268.
"Electronegativity Values from Thermochemical Data." Journal of Inorganic and Nuclear Chemistry, volume 17,
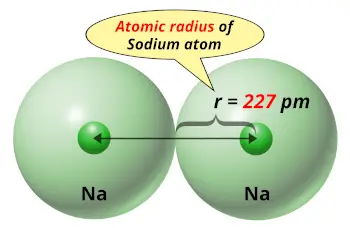
#Sodiums atomic radius free
"Electronegativity Is the Average One-Electron Energy of the Valence-Shell Electrons in Ground-State Free Atoms." Journal of New York: Oxford University Press, 1992.Īllen, Leland C. Next to a value above to see complete citation information for that entry)Īlbright, Thomas A., and Jeremy K.


 0 kommentar(er)
0 kommentar(er)
